What Does The Lysosomes Do In An Animal Cell
| Cell biology | |
|---|---|
| Animal cell diagram | |
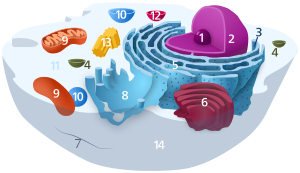 Components of a typical fauna jail cell:
|
A lysosome () is a membrane-leap organelle found in many animal cells.[one] They are spherical vesicles that contain hydrolytic enzymes that can intermission down many kinds of biomolecules. A lysosome has a specific composition, of both its membrane proteins, and its lumenal proteins. The lumen's pH (~4.five–v.0)[2] is optimal for the enzymes involved in hydrolysis, analogous to the action of the stomach. Besides degradation of polymers, the lysosome is involved in various cell processes, including secretion, plasma membrane repair, apoptosis, cell signaling, and energy metabolism.[3]

Lysosomes assimilate materials taken into the prison cell and recycle intracellular materials. Stride one shows fabric entering a food vacuole through the plasma membrane, a procedure known as endocytosis. In step ii a lysosome with an agile hydrolytic enzyme comes into the flick as the nutrient vacuole moves abroad from the plasma membrane. Stride three consists of the lysosome fusing with the food vacuole and hydrolytic enzymes entering the food vacuole. In the concluding step, footstep four, hydrolytic enzymes assimilate the nutrient particles.[4]
Lysosomes human action every bit the waste material disposal system of the cell past digesting used materials in the cytoplasm, from both inside and outside the cell. Material from outside the cell is taken up through endocytosis, while material from the inside of the cell is digested through autophagy.[v] The sizes of the organelles vary greatly—the larger ones can be more than than 10 times the size of the smaller ones.[6] They were discovered and named by Belgian biologist Christian de Duve, who somewhen received the Nobel Prize in Physiology or Medicine in 1974.
Lysosomes are known to contain more than than 60 different enzymes, and take more l membrane proteins.[vii] [8] Enzymes of the lysosomes are synthesised in the rough endoplasmic reticulum and exported to the Golgi appliance upon recruitment by a complex composed of CLN6 and CLN8 proteins.[nine] [10] The enzymes are trafficked from the Golgi apparatus to lysosomes in small-scale vesicles, which fuse with larger acidic vesicles. Enzymes destined for a lysosome are specifically tagged with the molecule mannose half-dozen-phosphate, then that they are properly sorted into acidified vesicles.[11] [12]
In 2009, Marco Sardiello and co-workers discovered that the synthesis of most lysosomal enzymes and membrane proteins is controlled past transcription factor EB (TFEB), which promotes the transcription of nuclear genes.[xiii] [14] Mutations in the genes for these enzymes are responsible for more than 50 different human genetic disorders, which are collectively known every bit lysosomal storage diseases. These diseases result from an accumulation of specific substrates, due to the inability to break them downwards. These genetic defects are related to several neurodegenerative disorders, cancers, cardiovascular diseases, and aging-related diseases.[15] [16] [17]
Discovery [edit]

TEM views of various vesicular compartments. Lysosomes are denoted by "Ly". They are dyed night due to their acerbity; in the middle of the top image, a Golgi Apparatus can be seen, distal from the cell membrane relative to the lysosomes.
Christian de Duve, the chairman of the Laboratory of Physiological Chemistry at the Cosmic University of Louvain in Belgium, had been studying the mechanism of action of a pancreatic hormone insulin in liver cells. By 1949, he and his team had focused on the enzyme chosen glucose 6-phosphatase, which is the beginning crucial enzyme in carbohydrate metabolism and the target of insulin. They already suspected that this enzyme played a primal role in regulating blood saccharide levels. However, even later on a series of experiments, they failed to purify and isolate the enzyme from the cellular extracts. Therefore, they tried a more backbreaking procedure of prison cell fractionation, past which cellular components are separated based on their sizes using centrifugation.
They succeeded in detecting the enzyme activeness from the microsomal fraction. This was the crucial step in the serendipitous discovery of lysosomes. To estimate this enzyme activeness, they used that of the standardized enzyme acid phosphatase and found that the activity was only 10% of the expected value. Ane twenty-four hours, the enzyme activity of purified jail cell fractions which had been refrigerated for v days was measured. Surprisingly, the enzyme action was increased to normal of that of the fresh sample. The result was the same no matter how many times they repeated the interpretation, and led to the conclusion that a membrane-like barrier limited the accessibility of the enzyme to its substrate, and that the enzymes were able to lengthened later on a few days (and react with their substrate). They described this membrane-like barrier as a "saclike construction surrounded by a membrane and containing acrid phosphatase."[xviii]
It became clear that this enzyme from the cell fraction came from bleary fractions, which were definitely cell organelles, and in 1955 De Duve named them "lysosomes" to reverberate their digestive backdrop.[19] The same year, Alex B. Novikoff from the University of Vermont visited de Duve's laboratory, and successfully obtained the first electron micrographs of the new organelle. Using a staining method for acid phosphatase, de Duve and Novikoff confirmed the location of the hydrolytic enzymes of lysosomes using low-cal and electron microscopic studies.[20] [21] de Duve won the Nobel Prize in Physiology or Medicine in 1974 for this discovery.
Originally, De Duve had termed the organelles the "suicide bags" or "suicide sacs" of the cells, for their hypothesized role in apoptosis.[22] However, it has since been concluded that they just play a minor part in prison cell death.[23]
Function and construction [edit]
Lysosomes contain a diverseness of enzymes, enabling the cell to suspension down various biomolecules information technology engulfs, including peptides, nucleic acids, carbohydrates, and lipids (lysosomal lipase). The enzymes responsible for this hydrolysis crave an acidic surround for optimal activity.
In addition to being able to pause down polymers, lysosomes are capable of fusing with other organelles & digesting big structures or cellular debris; through cooperation with phagosomes, they are able to comport autophagy, clearing out damaged structures. Similarly, they are able to break downwards virus particles or bacteria in phagocytosis of macrophages.
The size of lysosomes varies from 0.1 μm to one.2 μm.[24] With a pH ranging from ~iv.v–v.0, the interior of the lysosomes is acidic compared to the slightly basic cytosol (pH 7.two). The lysosomal membrane protects the cytosol, and therefore the rest of the cell, from the degradative enzymes within the lysosome. The prison cell is additionally protected from whatsoever lysosomal acrid hydrolases that drain into the cytosol, equally these enzymes are pH-sensitive and practice non role well or at all in the alkaline environment of the cytosol. This ensures that cytosolic molecules and organelles are not destroyed in example in that location is leakage of the hydrolytic enzymes from the lysosome.
The lysosome maintains its pH differential past pumping in protons (H+ ions) from the cytosol across the membrane via proton pumps and chloride ion channels. Vacuolar-ATPases are responsible for transport of protons, while the counter transport of chloride ions is performed past ClC-7 Cl−/H+ antiporter. In this way a steady acidic environment is maintained.[25] [26]
It sources its versatile chapters for deposition past import of enzymes with specificity for dissimilar substrates; cathepsins are the major form of hydrolytic enzymes, while lysosomal alpha-glucosidase is responsible for carbohydrates, and lysosomal acid phosphatase is necessary to release phosphate groups of phospholipids.
Formation [edit]

The lysosome is shown in purple, as an endpoint in endocytotic sorting. AP2 is necessary for vesicle formation, whereas the mannose-6-receptor is necessary for sorting hydrolase into the lysosome'south lumen.
Many components of animal cells are recycled past transferring them within or embedded in sections of membrane. For instance, in endocytosis (more than specifically, macropinocytosis), a portion of the jail cell'southward plasma membrane pinches off to grade vesicles that will somewhen fuse with an organelle inside the cell. Without active replenishment, the plasma membrane would continuously subtract in size. It is thought that lysosomes participate in this dynamic membrane substitution system and are formed by a gradual maturation process from endosomes.[27] [28]
The production of lysosomal proteins suggests one method of lysosome sustainment. Lysosomal protein genes are transcribed in the nucleus in a process that is controlled by transcription cistron EB (TFEB).[xiv] mRNA transcripts exit the nucleus into the cytosol, where they are translated past ribosomes. The nascent peptide bondage are translocated into the rough endoplasmic reticulum, where they are modified. Lysosomal soluble proteins leave the endoplasmic reticulum via COPII-coated vesicles afterwards recruitment by the EGRESS complex (EastR-to-Golgi relaying of due eastnzymes of the lysosomal southwardystem), which is composed of CLN6 and CLN8 proteins.[9] [x] COPII vesicles and so deliver lysosomal enzymes to the Golgi apparatus, where a specific lysosomal tag, mannose 6-phosphate, is added to the peptides. The presence of these tags permit for binding to mannose half-dozen-phosphate receptors in the Golgi appliance, a phenomenon that is crucial for proper packaging into vesicles destined for the lysosomal organisation.[29]
Upon leaving the Golgi apparatus, the lysosomal enzyme-filled vesicle fuses with a late endosome, a relatively acidic organelle with an guess pH of 5.5. This acidic surroundings causes dissociation of the lysosomal enzymes from the mannose vi-phosphate receptors. The enzymes are packed into vesicles for further transport to established lysosomes.[29] The late endosome itself can somewhen abound into a mature lysosome, as evidenced by the transport of endosomal membrane components from the lysosomes back to the endosomes.[27]
Pathogen entry [edit]

Cholera gaining entry into a cell via endocytosis.
Every bit the endpoint of endocytosis, the lysosome also acts as a safeguard in preventing pathogens from being able to reach the cytoplasm before beingness degraded. Pathogens oftentimes hijack endocytotic pathways such every bit pinocytosis in order to gain entry into the cell. The lysosome prevents easy entry into the jail cell by hydrolyzing the biomolecules of pathogens necessary for their replication strategies; reduced Lysosomal activity results in an increase in viral infectivity, including HIV.[30] In add-on, AB5 toxins such as cholera hijack the endosomal pathway while evading lysosomal degradation.[xxx]
Clinical significance [edit]
Lysosomes are involved in a group of genetically inherited deficiencies, or mutations called lysosomal storage diseases (LSD), inborn errors of metabolism caused by a dysfunction of i of the enzymes. The rate of incidence is estimated to be 1 in 5,000 births, and the true figure expected to be higher every bit many cases are probable to exist undiagnosed or misdiagnosed. The primary cause is deficiency of an acrid hydrolase. Other weather are due to defects in lysosomal membrane proteins that fail to ship the enzyme, non-enzymatic soluble lysosomal proteins. The initial effect of such disorders is accumulation of specific macromolecules or monomeric compounds within the endosomal–autophagic–lysosomal system.[15] This results in abnormal signaling pathways, calcium homeostasis, lipid biosynthesis and degradation and intracellular trafficking, ultimately leading to pathogenetic disorders. The organs about affected are brain, viscera, bone and cartilage.[31] [32]
There is no directly medical handling to cure LSDs.[33] The most common LSD is Gaucher's disease, which is due to deficiency of the enzyme glucocerebrosidase. Consequently, the enzyme substrate, the fatty acid glucosylceramide accumulates, specially in white blood cells, which in turn affects spleen, liver, kidneys, lungs, encephalon and bone marrow. The disease is characterized by bruises, fatigue, anaemia, low blood platelets, osteoporosis, and enlargement of the liver and spleen.[34] [35] As of 2017, enzyme replacement therapy is available for treating 8 of the 50-60 known LDs.[36]
The most severe and rarely found, lysosomal storage illness is inclusion prison cell disease.[37]
Metachromatic leukodystrophy is another lysosomal storage disease that too affects sphingolipid metabolism.
Dysfunctional lysosome activeness is also heavily implicated in the biology of aging, and historic period-related diseases such as Alzheimer'south, Parkinson'southward, and cardiovascular disease. [38] [39]
Different enzymes present in Lysosomes [40] [edit]
| Sr. No | Enzymes | Substrate |
|---|---|---|
| one | Phosphates | |
| A- Acid phosphatase | Most phosphomonoesters | |
| B- Acid phosphodiesterase | Oligonucleotides and phosphodiesterase | |
| 2 | Nucleases | |
| A- Acrid ribonuclease | RNA | |
| B- Acid deoxyribonuclease | DNA | |
| 3 | Polysaccharides/ mucopolysaccharides hydrolyzing enzymes | |
| A- β-Galactosidase | Galactosides | |
| B- α-Glucosidase | Glycogen | |
| C- α-Mannosidase | Mannosides, glycoproteins | |
| D- β- Glucoronidase | Polysaccharides and mucopolysaccharides | |
| E- Lysozymes | Bacterial jail cell walls and mucopolysaccharides | |
| F- Hyaluronidase | Hyaluronic acids, chondroitin sulfates | |
| H- Arylsulphatase | Organic sulfates | |
| iv | Proteases | |
| A- Cathepsin(due south) | Proteins | |
| B- Collagenase | Collagen | |
| C- Peptidase | Peptides | |
| 5 | Lipid degrading enzymes | |
| A- Esterase | Fatty acyl esters | |
| B- Phospholipase | Phospholipids | |
| vi | Sulfatases | |
| A- Arylsulfatase(A, B & G) | O- and Due north-Sulfate esters | |
| B- Glucosamine (N-acetyl)-6-Sulfatase/GNS | Glycosaminoglycans | |
| C- Iduronate 2-Sulfatase/IDS | O- and North-Sulfate esters |
Lysosomotropism [edit]
Weak bases with lipophilic properties accumulate in acidic intracellular compartments similar lysosomes. While the plasma and lysosomal membranes are permeable for neutral and uncharged species of weak bases, the charged protonated species of weak bases practice not permeate biomembranes and accumulate within lysosomes. The concentration within lysosomes may reach levels 100 to one thousand fold higher than extracellular concentrations. This phenomenon is called lysosomotropism,[41] "acid trapping" or "proton pump" effect.[42] The amount of accumulation of lysosomotropic compounds may be estimated using a cell-based mathematical model.[43]
A significant part of the clinically approved drugs are lipophilic weak bases with lysosomotropic properties. This explains a number of pharmacological properties of these drugs, such as high tissue-to-claret concentration gradients or long tissue emptying half-lives; these properties have been found for drugs such equally haloperidol,[44] levomepromazine,[45] and amantadine.[46] All the same, high tissue concentrations and long elimination one-half-lives are explained as well by lipophilicity and assimilation of drugs to fatty tissue structures. Important lysosomal enzymes, such as acid sphingomyelinase, may be inhibited by lysosomally accumulated drugs.[47] [48] Such compounds are termed FIASMAs (functional inhibitor of acrid sphingomyelinase)[49] and include for example fluoxetine, sertraline, or amitriptyline.
Ambroxol is a lysosomotropic drug of clinical use to care for conditions of productive cough for its mucolytic action. Ambroxol triggers the exocytosis of lysosomes via neutralization of lysosomal pH and calcium release from acidic calcium stores.[l] Presumably for this reason, Ambroxol was too found to improve cellular function in some disease of lysosomal origin such as Parkinson'due south or lysosomal storage disease.[51] [52]
Systemic lupus erythematosus [edit]
Impaired lysosome function is prominent in systemic lupus erythematosus preventing macrophages and monocytes from degrading neutrophil extracellular traps[53] and immune complexes.[54] [55] [56] The failure to dethrone internalized immune complexes stems from chronic mTORC2 activity, which impairs lysosome acidification.[57] As a result, allowed complexes in the lysosome recycle to the surface of macrophages causing an accumulation of nuclear antigens upstream of multiple lupus-associated pathologies.[54] [58] [59]
Controversy in botany [edit]
Past scientific convention, the term lysosome is applied to these vesicular organelles only in animals, and the term vacuole is applied to those in plants, fungi and algae (some animal cells besides accept vacuoles). Discoveries in plant cells since the 1970s started to challenge this definition. Plant vacuoles are found to exist much more diverse in construction and function than previously thought.[60] [61] Some vacuoles contain their ain hydrolytic enzymes and perform the archetype lysosomal activity, which is autophagy.[62] [63] [64] These vacuoles are therefore seen as fulfilling the part of the animal lysosome. Based on de Duve'due south description that "only when considered every bit function of a system involved directly or indirectly in intracellular digestion does the term lysosome describe a physiological unit", some botanists strongly argued that these vacuoles are lysosomes.[65] However, this is not universally accepted every bit the vacuoles are strictly not similar to lysosomes, such as in their specific enzymes and lack of phagocytic functions.[66] Vacuoles do not have catabolic activity and do not undergo exocytosis as lysosomes practice.[67]
Etymology and pronunciation [edit]
The word lysosome (, ) is New Latin that uses the combining forms lyso- (referring to lysis and derived from the Latin lysis, significant "to loosen", via Ancient Greek λύσις [lúsis]), and -some, from soma, "body", yielding "body that lyses" or "lytic body". The adjectival grade is lysosomal. The forms *lyosome and *lyosomal are much rarer; they use the lyo- form of the prefix merely are frequently treated by readers and editors equally mere unthinking replications of typos, which has no dubiousness been truthful as ofttimes as not.
Come across too [edit]
- Peroxisome
- Cathelicidin
- Antimicrobial peptides
- Innate immune system
References [edit]
- ^ Past convention similar cells in plants are chosen vacuoles, see § Controversy in phytology
- ^ Ohkuma S, Poole B (July 1978). "Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents". Proceedings of the National Academy of Sciences of the U.s.a. of America. 75 (seven): 3327–31. Bibcode:1978PNAS...75.3327O. doi:10.1073/pnas.75.7.3327. PMC392768. PMID 28524.
- ^ Settembre C, Fraldi A, Medina DL, Ballabio A (May 2013). "Signals from the lysosome: a control heart for cellular clearance and energy metabolism". Nature Reviews Molecular Cell Biological science. xiv (5): 283–96. doi:10.1038/nrm3565. PMC4387238. PMID 23609508.
- ^ Holtzclaw FW, et al. (2008). AP* Biology: to Back-trail Biology (8th AP ed.). Pearson Benjamin Cummings.
- ^ Underwood, Emily (2018). "When the brain's waste disposal system fails". Knowable Mag. doi:x.1146/knowable-121118-1.
- ^ Lüllmznn-Rauch R (2005). "History and Morphology of Lysosome". In Zaftig P (ed.). Lysosomes (Online-Ausg. 1 ed.). Georgetown, Tex.: Landes Bioscience/Eurekah.com. pp. 1–sixteen. ISBN978-0-387-28957-1.
- ^ Xu H, Ren D (2015). "Lysosomal physiology". Annual Review of Physiology. 77 (one): 57–80. doi:ten.1146/annurev-physiol-021014-071649. PMC4524569. PMID 25668017.
- ^ "Lysosomal Enzymes". www.rndsystems.com. R&D Systems. Retrieved 4 October 2016.
- ^ a b di Ronza A, Bajaj L, Sharma J, Sanagasetti D, Lotfi P, Adamski CJ, Collette J, Palmieri M, Amawi A, Popp 50, Chang KT, Meschini MC, Leung HE, Segatori L, Simonati A, Sifers RN, Santorelli FM, Sardiello Thou (Dec 2018). "CLN8 is an endoplasmic reticulum cargo receptor that regulates lysosome biogenesis". Nature Prison cell Biology. twenty (12): 1370–1377. doi:x.1038/s41556-018-0228-seven. PMC6277210. PMID 30397314.
- ^ a b Bajaj L, Sharma J, di Ronza A, Zhang P, Eblimit A, Pal R, Roman D, Collette JR, Booth C, Chang KT, Sifers RN, Jung SY, Weimer JM, Chen R, Schekman RW, Sardiello Yard (June 2020). "A CLN6-CLN8 circuitous recruits lysosomal enzymes at the ER for Golgi transfer". J Clin Invest. 130 (8): 4118–4132. doi:x.1172/JCI130955. PMC7410054. PMID 32597833.
- ^ Saftig P, Klumperman J (September 2009). "Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function". Nature Reviews Molecular Cell Biology. 10 (9): 623–35. doi:ten.1038/nrm2745. PMID 19672277. S2CID 24493663.
- ^ Samie MA, Xu H (June 2014). "Lysosomal exocytosis and lipid storage disorders". Journal of Lipid Research. 55 (6): 995–1009. doi:x.1194/jlr.R046896. PMC4031951. PMID 24668941.
- ^ Underwood, Emily (2018). "When the brain's waste disposal organization fails". Knowable Magazine. doi:10.1146/knowable-121118-ane.
- ^ a b Sardiello Thousand, Palmieri One thousand, di Ronza A, Medina DL, Valenza Thou, Gennarino VA, Di Republic of malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi Southward, Parenti G, Cattaneo E, Ballabio A (July 2009). "A factor network regulating lysosomal biogenesis and function". Science. 325 (5939): 473–7. Bibcode:2009Sci...325..473S. doi:10.1126/science.1174447. PMID 19556463. S2CID 20353685.
- ^ a b Platt FM, Boland B, van der Spoel Air-conditioning (November 2012). "The prison cell biological science of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction". The Journal of Cell Biology. 199 (5): 723–34. doi:10.1083/jcb.201208152. PMC3514785. PMID 23185029.
- ^ He LQ, Lu JH, Yue ZY (May 2013). "Autophagy in ageing and ageing-associated diseases". Acta Pharmacologica Sinica. 34 (5): 605–11. doi:10.1038/aps.2012.188. PMC3647216. PMID 23416930.
- ^ Carmona-Gutierrez, Didac; Hughes, Adam L.; Madeo, Frank; Ruckenstuhl, Christoph (1 Dec 2016). "The crucial impact of lysosomes in aging and longevity". Ageing Research Reviews. Lysosomes in Aging. 32: 2–12. doi:10.1016/j.arr.2016.04.009. ISSN 1568-1637. PMC5081277. PMID 27125853.
- ^ Susana Castro-Obregon (2010). "The Discovery of Lysosomes and Autophagy". Nature Education. 3 (nine): 49.
- ^ de Duve C (September 2005). "The lysosome turns fifty". Nature Cell Biology. 7 (nine): 847–9. doi:10.1038/ncb0905-847. PMID 16136179. S2CID 30307451.
- ^ Novikoff AB, Beaufay H, De Duve C (July 1956). "Electron microscopy of lysosomerich fractions from rat liver". The Journal of Biophysical and Biochemical Cytology. 2 (4 Suppl): 179–84. doi:10.1083/jcb.2.iv.179. PMC2229688. PMID 13357540.
- ^ Klionsky DJ (August 2008). "Autophagy revisited: a conversation with Christian de Duve". Autophagy. 4 (6): 740–three. doi:10.4161/auto.6398. PMID 18567941.
- ^ Hayashi, Teru, and others. "Subcellular Particles." Subcellular Particles., 1959.
- ^ Turk B, Turk Five (2009). "Lysosomes equally 'Suicide Bags' in Cell Death: Myth or Reality?". The Journal of Biological Chemistry. 284 (33): 21783–87. doi:10.1074/jbc.R109.023820. PMC2755904. PMID 19473965.
- ^ Kuehnel W (2003). Color Atlas of Cytology, Histology, & Microscopic Beefcake (4th ed.). Thieme. p. 34. ISBN978-one-58890-175-0.
- ^ Mindell JA (2012). "Lysosomal acidification mechanisms". Annual Review of Physiology. 74 (1): 69–86. doi:10.1146/annurev-physiol-012110-142317. PMID 22335796.
- ^ Ishida Y, Nayak S, Mindell JA, Grabe M (June 2013). "A model of lysosomal pH regulation". The Journal of General Physiology. 141 (6): 705–20. doi:ten.1085/jgp.201210930. PMC3664703. PMID 23712550.
- ^ a b Alberts B, et al. (2002). Molecular biology of the cell (quaternary ed.). New York: Garland Science. ISBN978-0-8153-3218-3.
- ^ Falcone S, Cocucci East, Podini P, Kirchhausen T, Clementi East, Meldolesi J (November 2006). "Macropinocytosis: regulated coordination of endocytic and exocytic membrane traffic events". Journal of Jail cell Science. 119 (Pt 22): 4758–69. doi:10.1242/jcs.03238. PMID 17077125.
- ^ a b Lodish H, et al. (2000). Molecular jail cell biology (fourth ed.). New York: Scientific American Books. ISBN978-0-7167-3136-8.
- ^ a b Wei BL, Denton PW, O'Neill East, Luo T, Foster JL, Garcia JV (May 2005). "Inhibition of lysosome and proteasome function enhances human immunodeficiency virus type 1 infection". Journal of Virology. 79 (9): 5705–12. doi:10.1128/jvi.79.9.5705-5712.2005. PMC1082736. PMID 15827185.
- ^ Schultz ML, Tecedor Fifty, Chang M, Davidson BL (August 2011). "Clarifying lysosomal storage diseases". Trends in Neurosciences. 34 (8): 401–x. doi:x.1016/j.tins.2011.05.006. PMC3153126. PMID 21723623.
- ^ Lieberman AP, Puertollano R, Raben North, Slaugenhaupt S, Walkley SU, Ballabio A (May 2012). "Autophagy in lysosomal storage disorders". Autophagy. 8 (5): 719–30. doi:10.4161/machine.19469. PMC3378416. PMID 22647656.
- ^ .Parenti Yard, Pignata C, Vajro P, Salerno M (January 2013). "New strategies for the treatment of lysosomal storage diseases (review)". International Journal of Molecular Medicine. 31 (i): 11–20. doi:10.3892/ijmm.2012.1187. PMID 23165354.
- ^ Rosenbloom BE, Weinreb NJ (2013). "Gaucher disease: a comprehensive review". Disquisitional Reviews in Oncogenesis. 18 (3): 163–75. doi:10.1615/CritRevOncog.2013006060. PMID 23510062.
- ^ Sidransky E (Oct 2012). "Gaucher affliction: insights from a rare Mendelian disorder". Discovery Medicine. 14 (77): 273–81. PMC4141347. PMID 23114583.
- ^ Solomon One thousand, Muro S (September 2017). "Lysosomal enzyme replacement therapies: Historical development, clinical outcomes, and future perspectives". Advanced Drug Delivery Reviews. 118: 109–134. doi:10.1016/j.addr.2017.05.004. PMC5828774. PMID 28502768.
- ^ Alberts, Bruce (2002). Molecular biology of the cell (fourth ed.). Garland Science. p. 744. ISBN978-0815340720.
- ^ Carmona-Gutierrez, Didac; Hughes, Adam 50.; Madeo, Frank; Ruckenstuhl, Christoph (1 December 2016). "The crucial touch of lysosomes in aging and longevity". Ageing Research Reviews. Lysosomes in Crumbling. 32: ii–12. doi:10.1016/j.arr.2016.04.009. ISSN 1568-1637. PMC5081277. PMID 27125853.
- ^ Finkbeiner, Steven (1 April 2019). "The Autophagy Lysosomal Pathway and Neurodegeneration". Cold Spring Harbor Perspectives in Biological science. 12 (3): a033993. doi:10.1101/cshperspect.a033993. ISSN 1943-0264. PMC6773515. PMID 30936119.
- ^ Pranav Kumar. (2013). Life Sciences : Fundamentals and practice. Mina, Usha. (third ed.). New Delhi: Pathfinder Academy. ISBN978-81-906427-0-five. OCLC 857764171.
- ^ de Duve C, de Barsy T, Poole B, Trouet A, Tulkens P, Van Hoof F (September 1974). "Commentary. Lysosomotropic agents". Biochemical Pharmacology. 23 (eighteen): 2495–531. doi:10.1016/0006-2952(74)90174-nine. PMID 4606365.
- ^ Traganos F, Darzynkiewicz Z (1994). "Lysosomal proton pump activity: supravital cell staining with acridine orange differentiates leukocyte subpopulations". Methods Prison cell Biol. 41: 185–94. doi:10.1016/S0091-679X(08)61717-3. PMID 7532261.
- ^ Trapp Southward, Rosania GR, Horobin RW, Kornhuber J (October 2008). "Quantitative modeling of selective lysosomal targeting for drug design". European Biophysics Journal. 37 (8): 1317–28. doi:10.1007/s00249-008-0338-iv. PMC2711917. PMID 18504571.
- ^ Kornhuber J, Schultz A, Wiltfang J, Meineke I, Gleiter CH, Zöchling R, Boissl KW, Leblhuber F, Riederer P (June 1999). "Persistence of haloperidol in human brain tissue". The American Periodical of Psychiatry. 156 (vi): 885–90. doi:10.1176/ajp.156.6.885. PMID 10360127. S2CID 7258546.
- ^ Kornhuber J, Weigmann H, Röhrich J, Wiltfang J, Bleich S, Meineke I, Zöchling R, Härtter Southward, Riederer P, Hiemke C (March 2006). "Region specific distribution of levomepromazine in the human brain". Periodical of Neural Transmission. 113 (3): 387–97. doi:10.1007/s00702-005-0331-3. PMID 15997416. S2CID 24735371.
- ^ Kornhuber J, Quack K, Danysz W, Jellinger K, Danielczyk W, Gsell West, Riederer P (July 1995). "Therapeutic brain concentration of the NMDA receptor antagonist amantadine". Neuropharmacology. 34 (vii): 713–21. doi:x.1016/0028-3908(95)00056-c. PMID 8532138. S2CID 25784783.
- ^ Kornhuber J, Tripal P, Reichel Grand, Terfloth 50, Bleich South, Wiltfang J, Gulbins E (January 2008). "Identification of new functional inhibitors of acid sphingomyelinase using a structure-holding-activity relation model". Journal of Medicinal Chemistry. 51 (two): 219–37. CiteSeerX10.one.1.324.8854. doi:10.1021/jm070524a. PMID 18027916.
- ^ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel K, Mühle C, Terfloth L, Groemer TW, Spitzer GM, Liedl KR, Gulbins Due east, Tripal P (2011). Riezman H (ed.). "Identification of novel functional inhibitors of acid sphingomyelinase". PLOS Ane. vi (eight): e23852. Bibcode:2011PLoSO...623852K. doi:ten.1371/journal.pone.0023852. PMC3166082. PMID 21909365.
- ^ Kornhuber J, Tripal P, Reichel M, Mühle C, Rhein C, Muehlbacher M, Groemer TW, Gulbins E (2010). "Functional Inhibitors of Acid Sphingomyelinase (FIASMAs): a novel pharmacological grouping of drugs with wide clinical applications". Cellular Physiology and Biochemistry. 26 (1): nine–20. doi:10.1159/000315101. PMID 20502000.
- ^ Fois G, Hobi Due north, Felder E, Ziegler A, Miklavc P, Walther P, Radermacher P, Haller T, Dietl P (December 2015). "A new role for an old drug: Ambroxol triggers lysosomal exocytosis via pH-dependent Ca²⁺ release from acidic Ca²⁺ stores". Jail cell Calcium. 58 (6): 628–37. doi:10.1016/j.ceca.2015.ten.002. PMID 26560688.
- ^ Albin RL, Dauer WT (May 2014). "Magic shotgun for Parkinson's affliction?". Brain. 137 (Pt 5): 1274–five. doi:ten.1093/brain/awu076. PMID 24771397.
- ^ McNeill A, Magalhaes J, Shen C, Chau KY, Hughes D, Mehta A, Foltynie T, Cooper JM, Abramov AY, Gegg M, Schapira AH (May 2014). "Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells". Brain. 137 (Pt 5): 1481–95. doi:10.1093/brain/awu020. PMC3999713. PMID 24574503.
- ^ Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann Yard, Voll RE, Zychlinsky A (May 2010). "Harm of neutrophil extracellular trap deposition is associated with lupus nephritis". Proceedings of the National Academy of Sciences of the Us. 107 (21): 9813–8. Bibcode:2010PNAS..107.9813H. doi:10.1073/pnas.0909927107. PMC2906830. PMID 20439745.
- ^ a b Monteith AJ, Kang S, Scott Due east, Hillman K, Rajfur Z, Jacobson Yard, Costello MJ, Vilen BJ (Apr 2016). "Defects in lysosomal maturation facilitate the activation of innate sensors in systemic lupus erythematosus". Proceedings of the National Academy of Sciences of the United states of America. 113 (15): E2142–51. Bibcode:2016PNAS..113E2142M. doi:10.1073/pnas.1513943113. PMC4839468. PMID 27035940.
- ^ Kavai M, Szegedi G (August 2007). "Immune complex clearance by monocytes and macrophages in systemic lupus erythematosus". Autoimmunity Reviews. 6 (7): 497–502. doi:10.1016/j.autrev.2007.01.017. PMID 17643939.
- ^ Kávai M, Csipö I, Sonkoly I, Csongor J, Szegedi GY (November 1986). "Defective allowed circuitous degradation by monocytes in patients with systemic lupus erythematosus". Scandinavian Periodical of Immunology. 24 (5): 527–32. doi:ten.1111/j.1365-3083.1986.tb02167.x. PMID 3787186. S2CID 23685272.
- ^ Monteith AJ, Vincent HA, Kang Due south, Li P, Claiborne TM, Rajfur Z, Jacobson M, Moorman NJ, Vilen BJ (July 2018). "mTORC2 Activity Disrupts Lysosome Acidification in Systemic Lupus Erythematosus by Impairing Caspase-one Cleavage of Rab39a". Journal of Immunology. 201 (two): 371–382. doi:ten.4049/jimmunol.1701712. PMC6039264. PMID 29866702.
- ^ Kang Southward, Rogers JL, Monteith AJ, Jiang C, Schmitz J, Clarke SH, Tarrant TK, Truong YK, Diaz M, Fedoriw Y, Vilen BJ (May 2016). "Apoptotic Debris Accumulates on Hematopoietic Cells and Promotes Disease in Murine and Human Systemic Lupus Erythematosus". Journal of Immunology. 196 (x): 4030–9. doi:x.4049/jimmunol.1500418. PMC4868781. PMID 27059595.
- ^ Kang South, Fedoriw Y, Brenneman EK, Truong YK, Kikly Chiliad, Vilen BJ (April 2017). "BAFF Induces Tertiary Lymphoid Structures and Positions T Cells within the Glomeruli during Lupus Nephritis". Journal of Immunology. 198 (7): 2602–2611. doi:10.4049/jimmunol.1600281. PMC5360485. PMID 28235864.
- ^ Marty F (April 1999). "Plant vacuoles". The Plant Cell. 11 (iv): 587–600. doi:10.2307/3870886. JSTOR 3870886. PMC144210. PMID 10213780.
- ^ Samaj J, Read ND, Volkmann D, Menzel D, Baluska F (August 2005). "The endocytic network in plants". Trends in Cell Biology. xv (viii): 425–33. doi:ten.1016/j.tcb.2005.06.006. PMID 16006126.
- ^ Matile, P (1978). "Biochemistry and Office of Vacuoles". Annual Review of Plant Physiology. 29 (one): 193–213. doi:10.1146/annurev.pp.29.060178.001205.
- ^ Moriyasu Y, Ohsumi Y (August 1996). "Autophagy in Tobacco Suspension-Cultured Cells in Response to Sucrose Starvation". Plant Physiology. 111 (4): 1233–1241. doi:x.1104/pp.111.4.1233. PMC161001. PMID 12226358.
- ^ Jiao BB, Wang JJ, Zhu XD, Zeng LJ, Li Q, He ZH (January 2012). "A novel poly peptide RLS1 with NB-ARM domains is involved in chloroplast deposition during leafage senescence in rice". Molecular Plant. five (one): 205–17. doi:10.1093/mp/ssr081. PMID 21980143.
- ^ Swanson SJ, Bethke PC, Jones RL (May 1998). "Barley aleurone cells comprise two types of vacuoles. Characterization Of lytic organelles by apply of fluorescent probes". The Establish Cell. 10 (5): 685–98. doi:x.2307/3870657. JSTOR 3870657. PMC144374. PMID 9596630.
- ^ Holtzman E (1989). Lysosomes. New York: Plenum Press. pp. seven, 15. ISBN978-0306-iv-3126-5.
- ^ De DN (2000). Plant Cell Vacuoles: An Introduction. Australia: Csiro Publishing. ISBN978-0-643-09944-9.
External links [edit]
| | Expect upwardly lysosome in Wiktionary, the gratis dictionary. |
-
 This article incorporates public domain textile from the NCBI document: "Science Primer".
This article incorporates public domain textile from the NCBI document: "Science Primer". - 3D structures of proteins associated with lysosome membrane
- Hide and Seek Foundation For Lysosomal Research
- Lysosomal Disease Network, a research consortium funded by the NIH through its NCATS/Rare Diseases Clinical Inquiry Network
- Cocky-Subversive Behavior in Cells May Hold Key to a Longer Life
- Mutations in the Lysosomal Enzyme–Targeting Pathway and Persistent Stuttering
- Animation showing how lysosomes are made, and their role
Source: https://en.wikipedia.org/wiki/Lysosome
Posted by: hardertraturness.blogspot.com

0 Response to "What Does The Lysosomes Do In An Animal Cell"
Post a Comment